AstraZeneca: Possible delay to Scotland's Covid-19 vaccine programme as under 30s offered alternative
It comes after the Medicines and Healthcare products Regulatory Agency (MHRA) announced that those aged 18 to 29 will be offered different vaccines where possible in the UK.
While incidents of blood clots are very rare, the regulator said in a press conference with the Joint Committee on Vaccination and Immunisation (JCVI), in the case of young adults with no underlying health conditions the risk between the virus and the vaccine is “very finely balanced”.
Advertisement
Hide AdAdvertisement
Hide AdThose under 30 who have been given a single dose of AstraZeneca vaccine should be given a second dose of the same vaccine, health officials said, and those over 30 who are offered the jag should accepted it without fear.

Figures suggest the risk of rare blood clot is the equivalent to four people out of every million who receive the vaccine.
The Scottish Government said it would follow the change in guidance from the JCVI, but stressed that the benefits of the AstraZeneca vaccine “outweigh the risks”.
Deputy Chief Medical Officer Nicola Steedman said the government is “currently considering” whether the change may impact the delivery programme in Scotland, but added that at the moment the nation is on track to give a first dose to all over 18s by the end of July.
The first doses of the Moderna vaccine were given in Scotland on Wednesday.
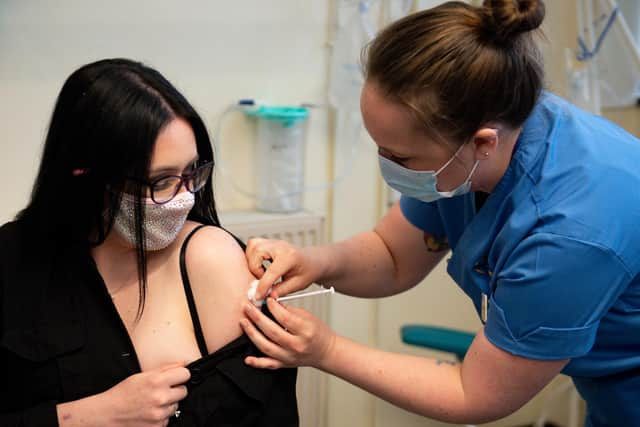

Due to cold storage requirements the jag is being delivered only at mass vaccination centres, beginning in Glasgow and with Edinburgh and Aberdeen to follow.
“Following the statements by the Medicines and Healthcare products Regulatory Agency (MHRA) and the Joint Committee on Vaccination and Immunisation (JCVI) we want to stress that the evidence shows that, overall, the benefits of the Oxford/AstraZeneca vaccine outweigh the risks and it continues to be a safe and effective vaccine,” said Dr Steedman.
“However, the Scottish Government will follow the JCVI recommendation to offer alternative vaccines to adults under 30 without underlying health conditions."
Advertisement
Hide AdAdvertisement
Hide AdShe added: “We continue to urge anyone offered a vaccination to take up their appointment. Everyone who has received their first dose of the AstraZeneca vaccine should receive their second dose as this gives greater and longer lasting protection against the virus.
"This is with the exception of the very few individuals who have had a blood clot with low platelet counts after their first injection, or an allergic reaction.”
Professor Wei Shen, chairman of the JCVI, said the recommendation was made “out of the utmost caution” rather than because of “any serious safety concerns”.
The MHRA’s chief executive, Dr June Raine, said there is a “reasonably plausible” link between the AstraZeneca jab and rare blood clots, but added that they are “extremely rare”.
Up to March 31, the MHRA received 79 reports of blood clots accompanied by low blood platelet count, all in people who had their first dose of the vaccine, out of around 20 million doses given.
Of these 79, a total of 19 people have died, three of whom were under 30, although it has not been established what the cause was in every case.
A message from the Editor:
Thank you for reading this article. We're more reliant on your support than ever as the shift in consumer habits brought about by coronavirus impacts our advertisers.
If you haven't already, please consider supporting our trusted, fact-checked journalism by taking out a digital subscription.
Comments
Want to join the conversation? Please or to comment on this article.