Cosmetic surgery is ripe for regulation – Jennifer Talbot
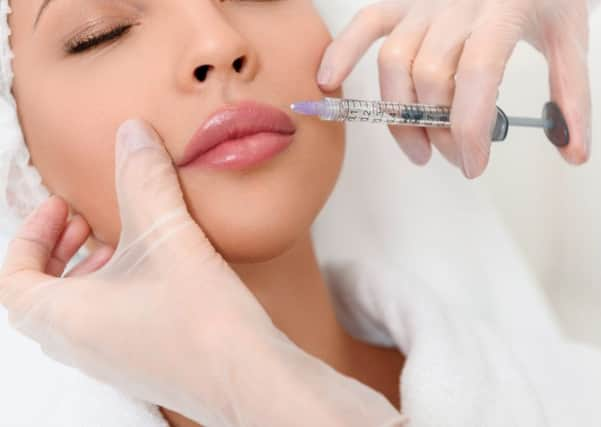

With an ever increasing focus on image and the pursuit of body “perfection”, for many people non-surgical cosmetic treatments have become a normal part of their beauty regime. In recent years, there has been a steep rise in the number of non-surgical cosmetic treatments performed throughout the UK.
Procedures once perceived as exotic are now a familiar offering on the high street; with salons and nail bars offering procedures including lip enhancements, derma fillers and botox injections. However, the familiarity that accompanies such “normalisation” masks potentially serious issues with significant legal consequences; putting customers and businesses alike at risk.
Advertisement
Hide AdAdvertisement
Hide AdThe cosmetic surgery industry has estimated UK-wide revenues of £3.6 billion; with non-surgical treatments such as fillers and botox accounting for nine out of 1ten procedures performed. However, there is a little-known lacuna in the current legal framework; non-medical professionals can carry out non-surgical cosmetic procedures entirely outwith the regulatory regime. The risks to consumers is clear; it is estimated that in 2019, over 80 per cent of consumers’ grievances with non-surgical cosmetic procedures lay with non-healthcare professionals.
This regulatory loophole is surprising and concerning given the nature (and potential consequences) of these procedures. Whilst these are non-surgical procedures, there is nonetheless great potential for harm and, possibly, limited scope for redress.
Calls for greater regulation and consumer protection have circulated for many years after attempts at self-regulation in the industry failed. In 2013, the UK-wide Keogh Review highlighted there was little regulation of this industry, and prompted calls for a new legislative framework. In 2015, the Scottish Cosmetic Interventions Expert Group (SCIEG) was established to investigate regulation of non-surgical cosmetic procedures. It recommended a phased approach to regulation, with phase one addressing independent clinics operated by healthcare professionals. Since 2016, such clinics have been regulated by Healthcare Improvement Scotland (HIS).
On 17 January, the Scottish Government launched a consultation seeking views on non-surgical procedures performed by non-healthcare professionals in non-healthcare premises. This followed recommendations from SCIEG to review the spectrum of compliance among practitioners, specifically those operating outwith the regulated healthcare sector.
The Government proposes that non-healthcare professionals who provide higher risk, non-surgical cosmetic procedures that pierce/penetrate the skin be regulated by licence.
The very need for the consultation may surprise readers, as there may be a reasonable expectation that such procedures, offered overtly, are regulated. Tattoo parlours and skin piercing are already subject to licensing and regulation. As with many trends, the statutory framework must evolve to ensure the law provides protection. The continued growth of this industry presents a challenge of constructing a regulatory framework which balances the benefits of regulation against the cost and practicality of compliance.
Whilst the consultation and proposals are welcome, there are many significant legal issues which must be addressed to increase accountability and ensure consumer safety.
Principally, there is the duty of care owed by practitioners to consumers. In addition to requirements regarding proficiency in performing treatments and ensuring standards of hygiene, there are issues concerning the minimum age of consumers (and age of consent), and identifying and dealing with vulnerable consumers. These procedures are principally concerned with body image. A practitioner’s duty of care must also involve detailed knowledge of the supply chain, and the manufacturer must be accountable for the quality and safety of the product. Consumers must be able to give informed consent and understand the significance of the procedure. The line between beauty treatment and cosmetic procedure can become blurred through normalisation. A key SCIEG recommendation was a ‘request for treatment’ agreement between provider and consumer; making contractual obligations and age restrictions more explicit.
Advertisement
Hide AdAdvertisement
Hide AdFor practitioners, there must also be greater clarity on the scope of regulations, and agreed terminology for qualifying procedures.
Another significant issue concerns insurance. There is no legal requirement for non-healthcare practitioners to hold insurance, or for provider organisations to have insurance against patient claims. All practitioners must be required to hold adequate insurance (relative to the procedure) to enable customers to seek redress following a failed procedure which, in some cases, could go beyond a refund.
All parties in the supply chain must be alert to the possibility of consumers raising claims. With the imminent introduction of class actions into Scots law, this could become easier and cheaper.
In consulting with a view to introducing regulations, Scotland is at the forefront of change in the UK. The consultation will run until 30 April and significant reform is anticipated.
Jennifer Talbot is a senior associate, DLA Piper