New research could reduce the amount of radiation required in medical imaging – Professor Gill Reid
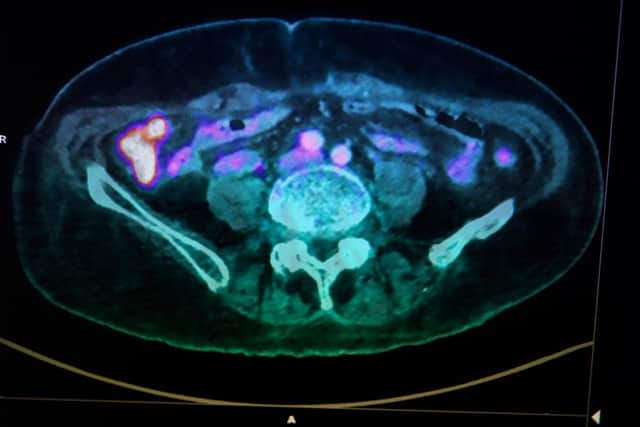
In nuclear medicine, for instance, use of radioactive isotopes is vital in allowing us to see inside the body to find out crucial information about diseases such as cancers and heart disease. One of the most often used is fluorine-18 (18F) – but the good news is only minute amounts of it are needed for medical imaging, even as little as 0.00000018 grams.
Fluorine-18’s biggest advantage is having a short, 110-minute half-life – the time taken for half its atoms to decay by releasing ‘positrons’, or tiny particles with the same mass as an electron and an equal but opposite charge. These can be utilised for medical imaging using a scanning technique called ‘positron emission tomography’ – a non-invasive procedure that injects radioactive tracer compounds (radiotracers), in this case containing fluorine-18, into patients.
Advertisement
Hide AdAdvertisement
Hide AdThe tracer molecules can be directed to specific organs or diseases, where the positrons are released, allowing doctors to take high-resolution 3D images of these areas from inside the body. Our labs at the University of Southampton – with collaborators at King’s College and Imperial College London, and GE HealthCare – are focussing on this field and right now are developing new molecules that can be ‘radio-fluorinated’ quickly and easily, and targeted to a particular region in the body to detect disease at an earlier stage. This will allow more effective intervention and better prospects for patient recovery.
We now hope to improve the process, using ‘molecular scaffolds’. Let me explain. As the fluorine-18 decays, the positrons travel only a few millimetres before they collide with an electron, producing two gamma-rays at 180 degrees to each other. These are then detected by a camera, allowing the 3D image of the target organ to be created.
The most widely used fluorine-18 molecule is ‘18-FDG’. However, since it accumulates in several organs at once, it lacks the selectivity and sensitivity needed for early disease detection. In Southampton, we are using metal compounds that we call ‘metal scaffolds’ to help form stable radiofluorine tracer molecules.
Due to that short half-life, the preparation of these 18F radiotracers must also be fast, ideally performed in water, and easily purified, before being injected into the patient. The new tracers should be effective at low concentrations to minimise the radiation dose needed. A further advantage of the metal scaffolds is that a small peptide can also be tethered, to direct the radiotracer towards specific target areas – again allowing far lower radioactive doses to be administered while giving higher imaging contrast.
These metal-fluorine combinations need to have other groups bonded to them to avoid further reactions that could release the radiofluorine, and for this we use molecular rings to wrap around the metal, providing an even better 18F binding site. Compounds with both gallium and iron are showing great promise, providing a hugely versatile approach for this ongoing research.

Gill Reid is professor of inorganic chemistry at the University of Southampton, president of the Royal Society of Chemistry, and a fellow of the Royal Society of Edinburgh. This research is funded by the Engineering and Physical Sciences Research Council (EPSRC) under the Mithras Programme. The RSE, Scotland's national academy, is on Twitter at @RoyalSocEd
Comments
Want to join the conversation? Please or to comment on this article.