Call for Scottish volunteers to trial first plant-based Covid-19 vaccine
Canada-based pharmaceutical company Medicago has launched phase three trials of its vaccine candidate, supported by the National Institute for Health Research (NIHR), NHS Research Scotland (NRS) and Health and Care Research Wales.
In the next month the company will recruit 1,500 volunteers across the UK, with a branch of the study based in Aberdeen.
Advertisement
Hide AdAdvertisement
Hide AdVolunteers between the age of 18 and 39 will be given two doses of the plant-based vaccine, 21 days apart, as well as two doses of a placebo in a separate phase of the programme.
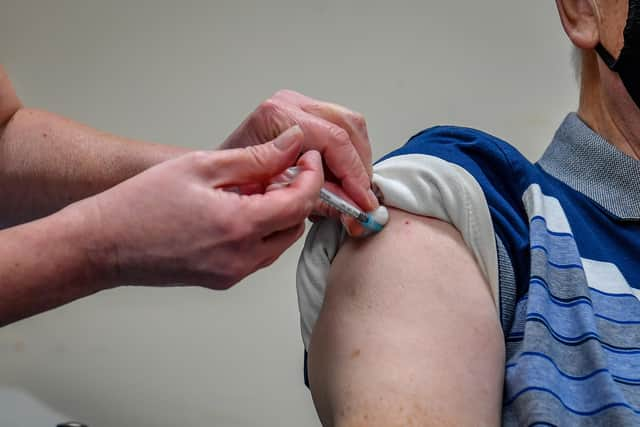

Responses will be studied for 12 months after their last vaccination.
The study is also taking place in Canada, the US and Latin America, as well as other sites across Europe.
Canada’s health regulator has recently begun a rolling review of the vaccine.
Chief Investigator for the study, Dr Chrissie Jones, associate professor in paediatric infectious diseases at Southampton University, said: "Clinical trials of Covid-19 vaccines are still needed in the UK to ensure that we have access to a range of different vaccines which are safe and effective.
"The Medicago Covid-19 vaccine candidate is developed within plants which produce non-infectious versions of the virus.”
Dr Roy Soiza, consultant physician at Aberdeen Royal Infirmary, said: “Clinical studies into Covid-19 vaccines remain critical to help find several safe and effective candidates to help protect us all. Volunteers in the Grampian region are still needed to help carry out these studies.
"We had an overwhelming response to previous vaccines trials and encourage interested participants to contact us by email ([email protected]) to get more information about taking part in this Covid-19 vaccine study.”
Advertisement
Hide AdAdvertisement
Hide AdThe study comes after 450 people in Aberdeen were recruited to a phase three trial of the Novavax Covid-19 vaccine in October.
A call for volunteers was issued last week by Valneva, for participants in phase three trials of the vaccine being manufactured in Livingston.
Some 4,000 volunteers will be recruited to that study, which will give participants either a Valneva vaccine or an already-approved vaccine manufactured by AstraZeneca.
A message from the Editor:
Thank you for reading this article. We're more reliant on your support than ever as the shift in consumer habits brought about by coronavirus impacts our advertisers.
If you haven't already, please consider supporting our trusted, fact-checked journalism by taking out a digital subscription.
Comments
Want to join the conversation? Please or to comment on this article.
