Breast and kidney cancer drugs get approval for use across Scotland's NHS


The Scottish Medicines Consortium (SMC) announced it had accepted the use of the treatments today while it also rejected atezolizumab for tackling non-small cell lung cancer.
Many were given the green light following work carried out through the SMC's patient and clinician engagement (Pace) process.
Advertisement
Hide AdAdvertisement
Hide Ad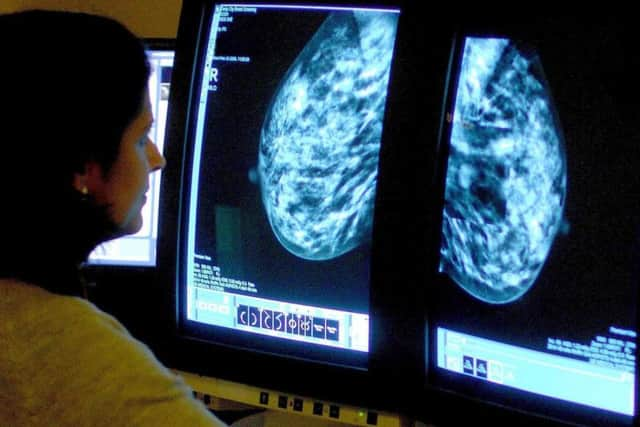

"From the testimonies given by patient groups and clinicians through our Pace process, we know that those with advanced breast cancer value the opportunity to have additional time with family and friends.
"We hope that our decision on ribociclib will be welcomed by them."
Ribociclib, used in combination with another medicine, was accepted for post-menopausal women living with the most common form of advanced breast cancer.
"Compared with fulvestrant alone, this combination treatment can offer patients invaluable extra time to live well before their disease progresses - time to live well and continue with day-to-day activities such as working for as long as possible.
"This treatment option could also help delay the need for chemotherapy and the often debilitating side effects it can bring, which is incredibly important for patients."
SMC has meanwhile also accepted Lenvatinib for the treatment of advanced renal cell carcinoma - kidney cancer - in those who have been previously treated with a type of medicine called a "vascular endothelial growth factor inhibitor".
Pentosan polysulfate sodium was approved through Pace for treating bladder pain syndrome, a condition which causes pain and a frequent, urgent need to pass urine.
Clostridium botulinum neurotoxin type A was accepted for tackling of chronic sialorrhoea - excessive drooling - due to neurological disorders.
The SMC also gave the green light for imiquimod to treat actinic keratosis, a precancerous, abnormal skin growth that can develop after too much exposure to sunlight.
