Scottish life sciences firm to break lucrative $500 million US market after 'novel' product approved by FDA
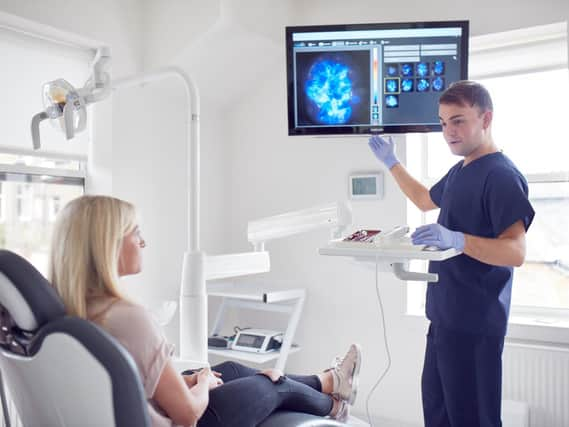

Calcivis is on the cusp of accessing the projected $500 million (£387m) American market following an approvable letter from the government-run Food and Drug Administration (FDA).
The life sciences firm, which last year raised £4.5m to further its push into the US, has developed a “novel” system for assessing, treating and preventing tooth decay.
Advertisement
Hide AdAdvertisement
Hide AdIt is now targeting a US test launch next year through its commercial base in Boston, Massachusetts, with the aim of creating a new preventive dentistry market segment.
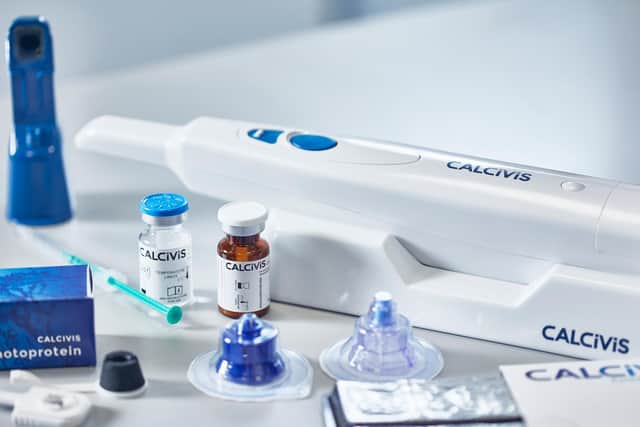

The Calcivis system enables dentists, for the first time, to differentiate in real-time between active and inactive lesions on tooth surfaces, which it claims can help dentists tailor care planning and allows for better preservation of patients’ original teeth.
Chief executive Adam Christie, said: “The receipt of an approvable letter from FDA is a very important step as we prepare to launch the Calcivis Imaging System in the world’s largest dentistry market.
“We are confident that Calcivis can help to establish a new standard of preventive care in US dentistry. Our technology helps dentists and patients to collaborate in promoting better oral health.”
Sarah Hardy, chief investment officer at business angel syndicate Archangels, which is a longstanding backer of the business along with the Scottish Investment Bank, said: “From the very beginning we have believed in the potential of Calcivis to make this step-change in preventive dentistry.
“Today’s announcement is a major corporate milestone in achieving this goal, which will ultimately allow the Calcivis Imaging System to help dentists in the US to better preserve their patients’ teeth.
