Life sciences firm Omega Diagnostics hails Covid test developments while flagging £8.7m impairment charge


The business also said it has shipped its first order for the test to detect if people have had the disease. Excluding the impairment, it expects core earnings for its latest financial year to come in slightly ahead of market expectations, at £850,000 to £900,000.
Omega flagged its signing of a longer-term supply agreement with Mologic, under which the latter will supply raw materials to enable Omega to make its CE-Marked Elisa antibody test.
Advertisement
Hide AdAdvertisement
Hide Ad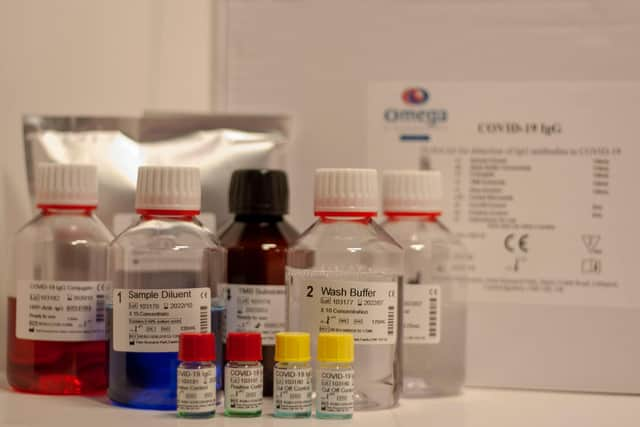

The test “will play a key part in identifying people that have antibodies demonstrating previous infection with Covid-19,” said Omega, which also specialises in testing related to HIV (its CD4 test), infectious diseases and food intolerance.
Omega also said it has shipped its first order for the Covid-19 Elisa test to Senegal worth about £100,000 and is talking to 15 countries, “which is expected to lead to orders in the near future”.
The business has also widened its collaboration with Mologic by signing another material transfer agreement, which will enable the Alva firm to broaden its growing portfolio of Covid-19 tests, using both its Scottish and English manufacturing sites “more effectively”.
Omega chief executive Colin King said: “We are pleased that Omega has been able to widen its collaboration with Mologic which should help to expand the number of Covid-19 tests that can be run both in centralised and decentralised settings, which, along with our Vistect CD4 tests, strengthens our position in global health.
“The decision to stop the development of further allergens has not been taken lightly but we recognise we can achieve better returns from directing our development efforts in other areas.”
FinnCap analysts said they have made minor mix changes to forecasts to reflect expected sales of Elisa Covid-19 sales in FY 2021, “which broadly offset the expected shortfall in food intolerance”. Adjusted core earnings are reduced by £200,000 to £1.6m, with adjusted pre-tax profit £300,000 higher at £700,000.
The developments come after Omega Diagnostics recently welcomed major progress by the consortium in which it is involved on developing a Covid-19 lateral flow antibody test that can be used at home.
A message from the Editor:
Thank you for reading this story on our website. While I have your attention, I also have an important request to make of you.With the coronavirus lockdown having a major impact on many of our advertisers - and consequently the revenue we receive - we are more reliant than ever on you taking out a digital subscription.Subscribe to scotsman.com and enjoy unlimited access to Scottish news and information online and on our app. With a digital subscription, you can read more than 5 articles, see fewer ads, enjoy faster load times, and get access to exclusive newsletters and content. Visit https://www.scotsman.com/subscriptions now to sign up.
Advertisement
Hide AdAdvertisement
Hide AdOur journalism costs money and we rely on advertising, print and digital revenues to help to support them. By supporting us, we are able to support you in providing trusted, fact-checked content for this website.
Joy Yates
Editorial Director
Comments
Want to join the conversation? Please or to comment on this article.