Scots drug maker to make cystic fibrosis inhaler
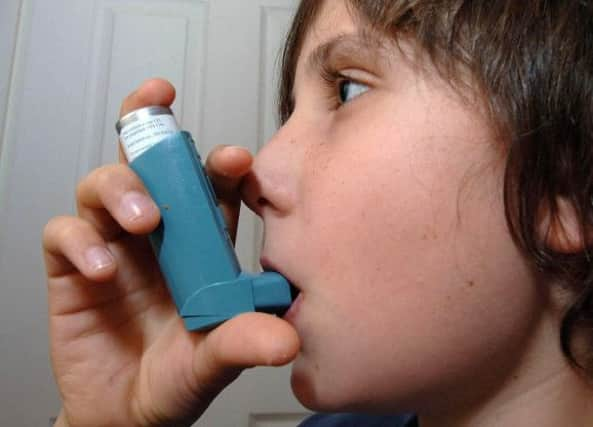

NovaBiotics signed the agreement with Bradford-based Crystec to develop a dry powder form of its Lynovex drug.
CF is an inherited disease that affects about 10,000 people in the UK and causes patients’ bodies to produce excessive mucus, which impacts the lungs and makes it hard to breathe.
Advertisement
Hide AdAdvertisement
Hide AdLynovex is an “orphan drug” candidate that breaks down mucus, disrupts bacterial films and acts as an antibacterial agent.
Orphan drugs are medicines that are often shunned by larger pharmaceutical companies because there is only a small market for them.
Deborah O’Neil, founder and chief executive at NovaBiotics, said: “Current treatments for CF are limited.
“Multiple therapies are often required but Lynovex could offer the possibility of a multi-functional, single treatment that addresses mucus production, bacterial infections and also persistent bacterial biofilms.
“I’m excited at the prospect of investigating how an inhaled form can achieve these clinical aims and, in doing so, could improve lung function in CF patients in the long term.”
Peter York, chief scientist at Crystec, added: “We are delighted to be supporting NovaBiotics on this project.
“We believe bringing together the therapeutic potential of Lynovex with the ‘best in class’ lung delivery performance of Crystec’s engineered particles could represent a ground-breaking treatment for this debilitating disease.”
NovaBiotics is also preparing to begin a phase-two clinical trial for Lynovex tablets that could be used to treat acute exacerbations of CF.
Advertisement
Hide AdAdvertisement
Hide AdThe work is being conducted in partnership with Aberdeen University and NHS Grampian.
NovaBiotics’ collaboration with the Cystic Fibrosis Trust to fund the phase-two trial will be highlighted as a case study at the UK Bioindustry Association’s annual parliament day next month at Westminster.
The meeting is described as a “flagship advocacy event, which represents the sector’s top policy needs to senior policymakers across government and sets the agenda for the year ahead”.
In August, NovaBiotics signed a deal with New York-listed Taro to develop a treatment for fungal nail infections, which could trigger tens of millions of dollars’ worth of royalty payments for the Aberdeen-based firm.
The world-wide nail fungus treatment market is expected to be worth more than £2 billion a year by 2022, according to figures from GlobalData.
NovaBiotics, which was launched in 2004, initially used its research to develop its nail fungus treatment before shifting its focus to more critical diseases such as CF.
The company’s board includes non-executive director Professor Andy Porter, the former chief scientific officer at Haptogen, who helped oversee its sale to Wyeth, one of the biggest deals to involve a Scottish life sciences company.
NovaBiotics has raised more than £10 million from private equity investors and Scottish Enterprise during the past decade and has considered floating on the stock market.