Omega Diagnostics raises cash as Alva firm cautions over test kit timings
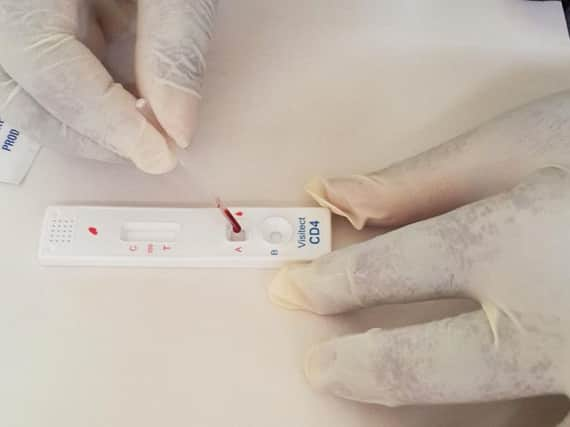

Reporting full-year results which showed an improving underlying financial position, the group said it was confident that it would receive the necessary approvals for its CD4 test but noted the existence of “material uncertainties with respect to timing of approvals and receipt of significant purchase orders”.
This could have an impact on short-term working capital requirements, it warned.
Shareholders have committed to pump £1.7m into the company, subject to approval at a forthcoming general meeting.
The investment would ensure that the group has access to sufficient working capital to continue to develop the commercialisation of both versions of its Visitect CD4 test.
Omega, which specialises in tests for allergies, infectious diseases and food intolerance, last month pushed back its full-year results citing “ongoing commercial discussions” as it unveiled new orders from China and Nigeria.
Releasing its delayed results for the year to the end of March, the firm said like-for-like revenues from continuing operations increased by 5 per cent to just under £8.8 million. Reported revenues were down by 28 per cent to about £9.8m, reflecting decisions last year to streamline the business, including the closure of its German allergy unit.
The group posted earnings before interest, tax, depreciation and amortisation (Ebitda) of £200,000 from continuing operations, compared with a loss of £800,000 a year earlier. Statutory profit for the year came in at £970,000, against losses of almost £7.3m previously.
The firm reported an adjusted loss before tax of £300,000, down from the £730,000 shortfall notched up in the prior 12-month period.
Interim non-executive chairman William Rhodes said: “We have made substantial, industry-leading advances in the area of CD4 testing, having achieved commercial launch of the first, and still only, handheld, lateral flow CD4 test and have rapidly progressed the advanced disease test to commercial launch as well.
“We are confident that we will receive the necessary approvals for CD4 but note the existence of material uncertainties with respect to timing of approvals and receipt of significant purchase orders and the resulting impact on short-term working capital requirements.”
He added: “We continue to explore unlocking the value within our three business units, whilst still managing to progress all three of them, namely, CD4 testing, allergy and food intolerance testing.”