New Ebola vaccine results show 100% success rate
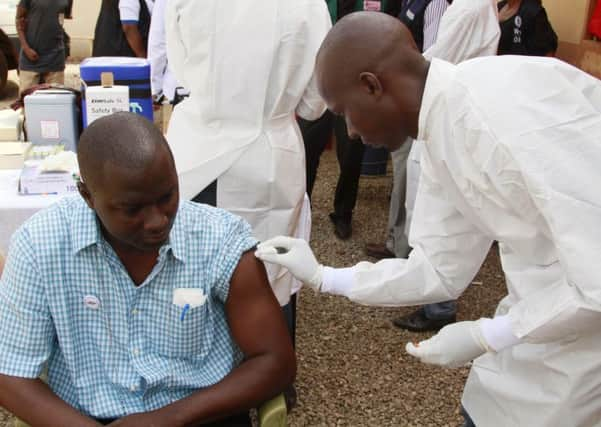

The experimental vaccine VSV-ZEBOV was found to be 100 per cent effective six days after it was administered to 7,651 people living in Guinea, where the virus has already claimed more than 2,500 lives.
It made no difference if people had the jab immediately or following a delay of three weeks after giving their consent to treatment.
Advertisement
Hide AdAdvertisement
Hide AdCritically, non-vaccinated individuals were also protected. A “ring vaccination” strategy – used in the past to eradicate smallpox – was adopted to create a buffer zone of protection by reducing the spread of the disease.
Dr Jeremy Farrar, director of Britain’s biggest research charity the Wellcome Trust, said: “Our hope is that this vaccine will now help bring this epidemic to an end and be available for the inevitable future Ebola epidemics. It should change how the world responds to emerging infectious disease threats.”
The trial was given the title “Ebola ca Suffit”, which means “Ebola this is Enough”.
It took place in Basse-Guinea, the only district of Guinea with new Ebola cases at the start of the study on 1 April. For every individual confirmed as being newly infected, researchers traced all the people he or she may have been in close contact with.
Adult contacts aged 18 and older who were not pregnant or breastfeeding were offered the vaccine. If consent was given, adults were randomised either to receive it immediately or after a delay. Comparisons between an active and “dummy” placebo vaccine could not be made for ethical reasons, since it would have meant condemning one group of participants to a high risk of death.
Under the ring vaccination strategy, reported in the Lancet, the jabs were followed up by monitoring the contacts, and contacts of contacts, of an immunised individual.
Dr Marie Paule Kieny, one of the researchers at the World Health Organisation in Geneva, Switzerland, said: “Before the trial started, in most [population] clusters there had been a series of Ebola cases over the weeks prior to randomisation.
“However, since the trial started, we have seen no new cases in vaccinated volunteers within ten days of vaccination, regardless of whether vaccination was immediate or delayed.”
Advertisement
Hide AdAdvertisement
Hide AdIn fact, the vaccine appeared to be offering protection in as little as six days.
A total of 16 new cases were recorded in the “delayed” vaccination clusters, which included unvaccinated contacts.
Taking account of untreated as well as treated individuals, the vaccine provided 75 per cent overall protection to communities participating in the trial.
“We don’t know how long the effects of the vaccine last, but the best use for this vaccine may be in ring-fencing suspected Ebola cases by vaccinating the people who have had close contact with Ebola patients,” Dr Kieny said.
VSV-ZEBOV was developed by the Public Health Agency of Canada and is licensed to NewLink Genetics and Merck.
The vaccine contains no live Ebola virus. It works by replacing a gene from a harmless virus with one encoding an Ebola virus surface protein. This is enough to prompt the immune system to launch a defensive response against Ebola.
Dr Ben Neuman, lecturer in virology at the University of Reading, said: “This is big news – the most promising medical development so far in the ongoing race to shut down Ebola.”